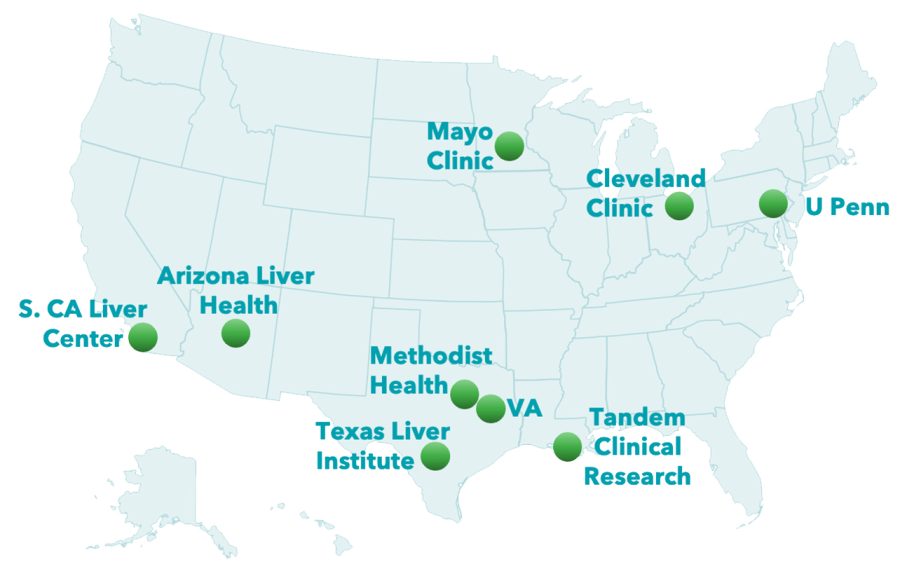
PharmaIN is currently enrolling patients in its ongoing Phase 1 clinical study evaluating PHIN-214 in patients with advanced cirrhosis.
The study is being conducted at various clinical sites across the United States. This single and multiple ascending dose (SAD and MAD) study is evaluating the safety, tolerability, and pharmacokinetics of PHIN-214 and establishing the maximum tolerated dose in patients with Child Pugh A and B Cirrhosis.
To learn more about PharmaIN’s ongoing clinical trial, please visit ClinicalTrials.gov at the following link: NCT05490888
Clinical Sites Currently Recruiting